Updated 9 April 2020
Anticipated Clinical Scenarios
Clinical Mechanical Ventilation 101
Minimum Parameter Set
Operating Modality
Additional Clinical Insights
Long Term Bag-Based Ventilation?
This section guides the engineering design with a focus on safety.
Caution: This section MUST be read and understood fully first. No engineering team should consider designing a ventilator without a clinician experienced in mechanical ventilation and respiratory management involved.
See Key Ventilation Specifications for a summary of ventilation issues discussed below.
The MIT Emergency Ventilator is intended for emergency use only when all available conventional invasive respiratory support has been exhausted. It should only be used in a clinical environment under careful monitoring by trained medical professionals. This has been developed by a team of physicians certified in Anesthesia and Critical Care, working with mechanical, electrical, and software engineers. There were two critical tasks which we started with:
- Identify potential use scenarios
- Define the minimum safe clinical functional requirements (specs)
To minimize time to bedside without compromising patient safety, the clinical functional requirements were distributed to a broad team of clinical advisors. In parallel, a peer-review process was used to identify what was felt to be the best design concept (in spirit, loosely based on a prior student project which was not clinically validated). Once a review of the clinical functional requirements was completed, it was distributed to the design and controls teams.
We hope that this website can act as a reference point to help others and encourage discussion. Also, it is intended as a resource for makers or manufacturers to access and utilize the latest design. As it stands, several important tasks remain:
- FDA review and feedback, work towards approval
- Long-term (days) porcine trials
- Implement design for manufacturing
- Logistics for manufacture, distribution, and quality controls
Anticipated Clinical Scenarios
Specific to the present COVID-19 pandemic, we anticipate the following scenarios in which an emergency mechanical ventilator could be safely used to provide respiratory support:
- A deteriorating COVID-19 patient, who is short of breath & hypoxic; hypoxemic respiratory insufficiency means they are not breathing well enough to adequately oxygenate their blood. Clinicians at this point can initiate respiratory support. An MIT Emergency Ventilator could provide basic respiratory support in this situation
- Worsening clinical status recognized when a patient develops Acute Respiratory Distress Syndrome (ARDS). An MIT Emergency Ventilator could be a bridging solution until a traditional ICU ventilator becomes available
- The patient will be intubated or have a tracheostomy (limited / no applicability to mask)
- Those patients are otherwise going to be sedated and paralyzed (invasive ventilation requires sedation, and paralysis will prevent patient-ventilator dyssynchrony if assist-control is not available)
- Ventilated patients required to leave the ICU for imaging or procedures can be supported with the MIT Emergency Ventilator, unless determined that the patient requires support outside its range.
A multidisciplinary team consisting of a physician, critical care nurse, and respiratory therapist should be available to monitor ventilated patients at all times. Additionally, a clinical lab capable of timely reporting of blood gases and other common ICU laboratory markers should be available to enable the clinical team to make appropriate decisions and adjustments.
Acute Respiratory Distress Syndrome
Those patients with ARDS would preferentially receive mechanical ventilation by standard ICU ventilators. A manual resuscitator is meant as a backup should institutions run out of traditional ventilators, and for patients with milder forms of lung disease that require less sophisticated modes and features.
Changes in lung mechanics (compliance) can be a result of acute and chronic lung conditions. In general, lung compliance is affected by a multitude of factors; in ARDS, fluid is present in the alveoli and/or interstitial space (between the alveoli and a capillary blood vessel) and results in changes in the diffusion of gases between the alveoli and blood vessel. Other conditions include:
- Any pathologies that cause fluid accumulation in the lung (‘wet lung’) through infectious, inflammatory, mechanical, or hydrostatic factors (pulmonary edema, TRALI, pneumonia, pneumonitis, diffuse alveolar hemorrhage, heart failure, cardiogenic shock, mitral valve regurgitation)
- Any pathology that causes fibrosis (scarring and thus stiffening – ‘stiff lung’) of the lung structure, otherwise known as the “parenchyma” (ARDS related, interstitial lung disease, sarcoidosis, idiopathic pulmonary fibrosis, radiation or chemotherapy-related, post pneumonia or hemothorax related trapped lung or lung fibrosis)
The safe limit for ventilation therapy has not yet been determined. In the life-and-death situation we are currently facing, this will give patients a chance until an ICU or OR ventilator becomes available. We are actively engaging with animal testing laboratories to determine what, if any, these limitations may be. Further, we plan to perform multi-day trials in pigs to evaluate the safety of longer-term MIT Emergency Ventilator use.
Clinical Mechanical Ventilation 101
If we boil down how a modern ICU ventilator works, there are three important parameters.
- Tidal volume (air delivered to the patient)
- Inspiratory phase start (“triggering”)
- Expiratory phase start (“cycling”)
Each of these values is first determined by the machine and healthcare operator. Adjustments are made in real-time to optimize the patient’s clinical status, as measured by checking lab draws and monitoring vital signs. The patient acts as a “built-in” sensor!
Tidal Volume: Volume-Control vs. Pressure-Control
Tidal volume, one can set a specific volume in milliliters or set an inspiratory pressure on the mechanical ventilator; tidal volume is often discussed and thought about as a value based on cc/kg of ideal body weight (see Equation 1). In Acute Respiratory Distress Syndrome (ARDS), patients’ tidal volumes are kept between 4 to 8 cc/kg. Here is a convenient chart (PDF) provided by ARDSNet with values for ideal or predicted body weight and different tidal volumes corresponding to the patient’s height.
Equation 1. Gender-specific formulas to calculate ideal body weight (courtesy: ARDSNet):
- Male Ideal Body Weight (kg) = 50 +[0.91 (height in cm – 152.4)]
- Female Ideal Body Weight (kg) = 45.5 +[0.91 (height in cm – 152.4)]
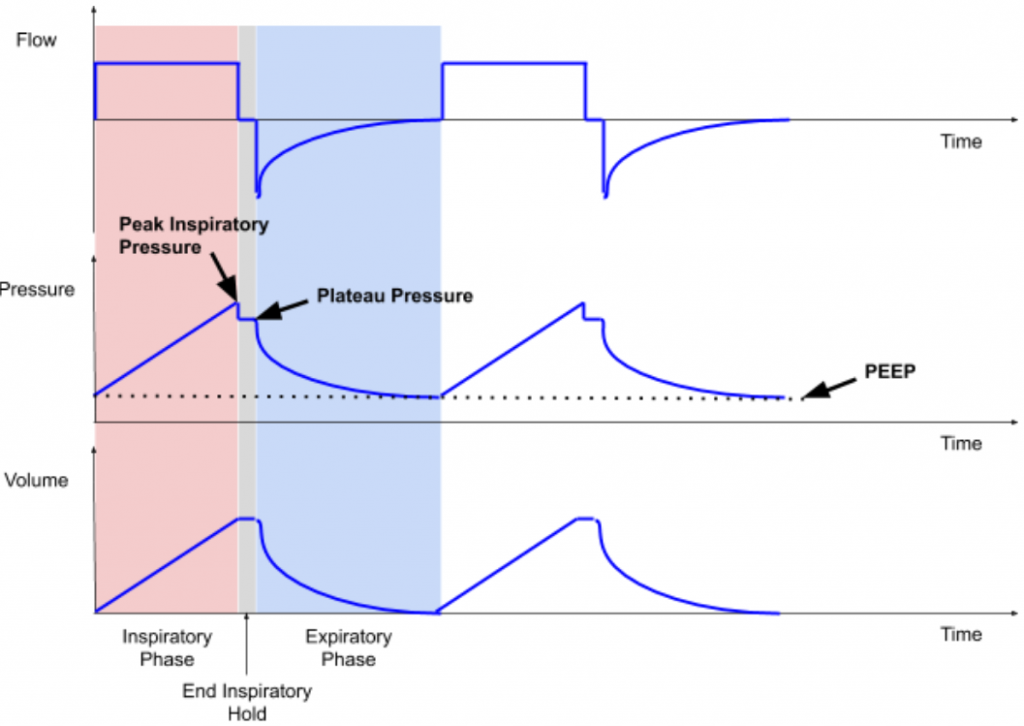
Volume control mode is just that: a clinician defines the tidal volume, see Figure 1. The machine will then try to deliver that volume with a uniform inspiratory flow rate, over a specified inspiratory time (see discussion on cycling). This is done regardless of how much pressure builds up in the lungs, referred to as peak inspiratory pressure (PIP). Modern ventilators have safety features to limit max pressures, which can result in damage to the lungs (a.k.a. barotrauma). Ventilators have the capability to perform an “end-inspiratory hold”, for a programmable duration over which the pressure in the circuit is recorded. This is called plateau pressure (Pplat). A volume-controlled breath cycle with inspiratory hold is illustrated in Figure 1.
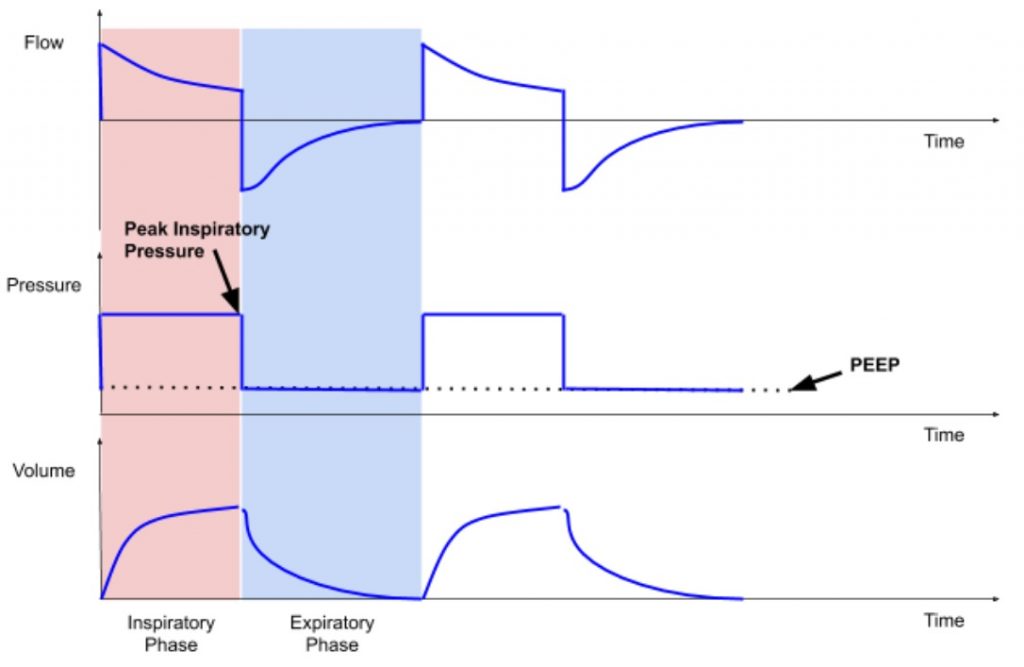
Pressure control mode utilizes pressure supplied by the ventilator, and the patient’s lung compliance and inspiratory time determine the volume of gas delivered (tidal volume), see Figure 2. As we are actively learning more about patients with COVID-19, what we do know is that there is an ARDS-like clinical picture. Therefore we know that in COVID-19 patients, the lung compliance changes with the disease course, and thus tidal volume will change with long-term use of pressure control ventilation.
This presents another branch point for granular clinical details: compliance can be further broken down into that of the upper and lower airways, see Figure 3. The upper airway consists of some structures bypassed by something like an endotracheal tube, namely the mouth, nose, oropharynx, and trachea. The lower airway consists of the bronchi (left and right mainstem, which further branches into secondary and tertiary bronchi, bronchioles, and alveoli). Compliance is also affected by the type of lung disease, grouped into restrictive or obstructive types, each further divided into extrinsic and intrinsic types. COVID-19 patients who develop ARDS have an intrinsic, restrictive disease which requires additional baseline pressure to help “prop” open alveoli to maintain gas exchange. This is achieved by positive end-expiratory pressure (PEEP).
Inspiratory phase start: time / pressure / flow triggering
Inspiratory phase can either be set to start at a regular interval by locking in a constant respiratory rate (e.g. time triggering) or have the ventilator sense the patient’s native inspiratory effort (with a pressure or flow sensor on the circuit), and time the start of the inspiratory phase according to the patient’s effort. This is analogous to oxygen pulse devices used by acrobatic plane pilots. Modern ICU ventilators can be set to trigger based on thresholds of flow (e.g. 1–4 L/min) or pressure (e.g. -1 to -5 cm H20) to initiate breaths. These are either inherent to a specific built-in ventilation mode (SIMV, PS, CPAP, etc; outside the present scope), or set by the clinical operator (respiratory therapist, nurse, CRNA, physician, etc).
Here, it should be noted that there is a difference between ICU ventilators and OR ventilators: ICU ventilators tend to be more advanced and are designed to care for patients who may require support for days, or weeks. OR ventilators are simpler and generally used on healthier patients for shorter periods of time (minutes to hours).
Expiratory phase start: time / volume / flow / pressure cycling
The start of the expiratory phase can be determined by different variables: time, volume, flow, and pressure. Inspiratory phase duration can be programmed and expiration starts immediately after the time for inspiration is complete; this is called “time cycling.” In volume control, inspiration stops after the target inspiratory volume has been delivered; this is called “volume cycling.” When inspiratory flow can be sensed, mechanical ventilator breath can switch from inspiration to expiration when the inspiratory flow reaches 10–25% of peak inspiratory flow; this is called “flow cycling.” Lastly, inspiration can be cycled into exhalation when a threshold pressure is reached. For instance, if a patient coughs and becomes asynchronous with the ventilator, the airway pressure increases dramatically. This can be dangerous to the patient as ventilation is not effective when the patient is “fighting the vent.” In this state, the ventilator switches inspiration to the exhalation phase and usually concurrently triggers the high-pressure alarm. This is called “pressure cycling.”
Additionally, in considering a single breath “cycle”, the ratio of time spent breathing in (inspiratory) vs. exhaling (expiratory) is important to consider as more time is required to fully exhale and prevent over-inflation (i.e. breath stacking or auto-PEEP). Inspiratory phase duration can be adjusted by altering the inspiratory to expiratory (I:E) ratio on the ventilator when a specific respiratory rate (bpm) is being used.
Positive end-expiratory pressure (PEEP) is applied in order to maintain an ‘open lung’, prevent alveolar collapse and thus improve gas exchange and minimize atelectrauma (repeated opening and collapse of alveoli “atelectasis” can also cause damage; the result of which is referred to as atelectrauma). In addition, due to the inhomogeneity of the lung tissues, positive pressure ventilation may lead to regional overdistention of alveoli (volutrauma and barotrauma), which can impair gas exchange and possibly further injure the diseased lung. The regional differences in lung compliance are dynamic and significantly change throughout a patient’s hospital course.
Depending on whether the patient or the machine determines each of the above parameters, different ventilatory modes are created. Some examples include volume control, pressure control, assist control, pressure support, SIMV, and spontaneous modes. Full-feature ICU ventilators have other ventilatory modes available that better serve longer-term mechanical ventilation strategies.
Minimum Parameter Set
An automated mechanical ventilator should initially operate in volume control mode, with an initial rate, adjusting minute ventilation as the patient’s homeostasis is optimized with vital signs and lab draws. We envision two versions:
- Volume Control: closed-loop delivery of a given tidal volume; closed-loop implies airway pressure sensing use for safety.
- Assist Control: the system will sense airway pressure fluctuations, and supports patient-initiated breaths, and then recognizes and allows exhalation.
In the simplest implementation, the system will be tuned using direct clinical observation and laboratory studies. This can serve as a transient device (e.g. for transport or bridge to more advanced ventilator) or as a definitive ventilator once demand outpaces available resources.
The minimum required hospital-supplied components are below, and harnesses existing infrastructure to increase scale-ability:
- Manual resuscitator “Ambu” bag: different configurations; it is recommended that a pop off (pressure release) valve and PEEP valve (listed in alphabetical order, with no preference given for any model) are included in any circuit. Suggested models include:
- Ambu SPUR II Disposable Resuscitator (need to purchase PEEP valve adapter separately)
- CareFusion AirLife Adult Disposable Self Inflating Resuscitation Device (need to buy PEEP valve adapter separately)
- Teleflex Lifesaver Disposable Manual Resuscitator (Catalog #5374; pop-off valve and PEEP included)
- VBM Germany PVC Resuscitator Set (40 cm H2O pop off valve and PEEP valve included)
- PEEP valve can be purchased separately if needed:
- Ambu PEEP Valves
- CareFusion AirLife Adjustable PEEP Valves
- Endotracheal (ET) and/or Tracheostomy tubes:
- Follows ISO standards and have standardized connectors
- Proper breathing circuit with proper valve mechanism at the patient end to minimize dead space and rebreathing of CO2
- Short flexible connector to connect end of breathing circuit to ET/trach
- AirLife Omni-Flex Patient Connector
- Oxygen / Air mixer if available (to adjust FiO2)
- HEPA filter to remove virus particles from expired gases (optional; likely not required if patient is in isolation).
- Thermovent HEPA Low Deadspace Heat and Moisture Exchange Filter
Minimum Performance Parameters:
| Parameter | Value or Range | Note |
| Modes | Volume Control Assist Control Fail-safe | Recognize if patient stops breathing –> switch to default |
| Tidal volume | 200 – 800 mL 6 mL or less / kg (ideal patient weight) as start point | Must be adjustable |
| Rate | 8 – 40 or 10 – 40 breaths per minute | Must be adjustable |
| PEEP[1] (part) | 5 – 20 cm H20 | Must be adjustable |
| Plateau pressure | Threshold: 40 – 60 cm H20 ; achieve with valve (part) | Usually fixed depending on Ambu bag type |
| I/E (inspiratory/ expiratory ratio) | 1:2; range of 1:1 – 1:4 COVID-19 patients are frequently requiring 1:3 and higher | Adjustable |
| Expired filtration | HEPA available as a component | Must be in line |
| Inspired filtration | HEPA available as a component | Must be in line |
| Inspired humidification | Combine with outgoing (self-humidification) | Recommended |
| Assist Control (breath detection or tigger sensitivity) | Sense pressure of -1 to -5 cm H20 | Recommended – requires pressure transducer in design |
| FiO2 | 30%-100% | Recommended |
| Peak inspiratory pressure (PIP) | Will be set by pop off valve threshold in initial product. If pressure transducer is used to continuously measure airway pressure, can program to limit PIP | Fixed or Adjustable – requires pressure transducer in design |
[1] Not recommended to use gravity driven PEEP valve
Additional considerations are not defined based on things like necessary electrical supply or gas supply. We are assuming that units will be manufactured with good manufacturing practices and power provided with off-the-shelf or similar technology. Further, operating in an environment under the supervision of a physician gives the highest chance for safe treatment.
Operating Modality
- Patients will be intubated or have a tracheostomy
- PEEP pressure can be adjusted as per clinical judgment (typically started between 5-10cm H20).
- Respiratory rate is adjusted according to clinical needs. This will be available digitally or via mechanical adjustment. If not digitally set, the clinician will time the device using their watch.
- I/E ratio set digitally or via a mechanical adjustment, if available, but it can be fixed.
- Oxygen supply is connected to the bag. The inhaled oxygen fraction can be adjusted using a gas flow blender (mixing 100% oxygen & air (21% oxygen) according to the patient’s clinical status).
- ABVV connected to patient, patient observed and settings adjusted based on SpO2, clinical evaluation, etc.
- The tidal volume is ramped up by observing bag compression as per clinical judgment. Later the system can be calibrated for a particular bag brand and tidal volume will be initially set to the value closest to 6 cc/kg ideal body weight.
Additional Clinical Insights
- The simplest machine is capable of operating in volume control mode, which does not incorporate breath sensing, is applicable only to sedated & paralyzed patients.
- Non-paralyzed patients are unlikely to tolerate a machine that is not able to detect inspiration. Opinions differ, and a one-way inspiratory valve may be OK in a non-paralyzed patient: https://www.harvardapparatus.com/one-way-respiratory-valves.html
- When a patient does spontaneously try to breath, air must flow to avoid negative pressure generation, which in turn could lead to pulmonary edema and further worsen lung compliance and gas exchange. Most Ambu-bags have this as a default feature.
- An over-pressure (PIP) value is required, with an active alert based on a pressure transducer. An acceptable solution is to reduce inspired volume, and most patients will tolerate this.
- Adjustment of I/E ratio improves flexibility, but it is not essential and easier to do with digital control.
- Acute Respiratory Distress Syndrome (ARDS): based on clinical reports, COVID-19 patients who are intubated for invasive mechanical ventilation initially have relatively normal compliance, and normal driving pressures can be used (plateau pressure minus PEEP, with a goal of < 15cm H20). Lung compliance, however, often decreases with worsening ARDS.
- Must be labelled with a warning that the MIT Emergency Ventilator does not have standard sensing, alert, and safety capabilities.
- Even if limited oxygen is available, the MIT Emergency Ventilator can protect patients from self-inflicted lung injury (SILI). This is lung trauma caused by erratic respiratory patterns, large intrathoracic pressure swings, and shear injury between heterogeneous lung parenchyma in dyspneic patients with diseased lungs.
Long Term Bag-Based Ventilation?
The suitability of manual resuscitator based ventilation for intubated patients and especially for those with COVID-19 remains an open question, on that we expect to be discussed in the months ahead. For the moment, we can only cite short term, more anecdotal evidence.
- Six Hours of Manual Ventilation With a Bag-Valve-Mask Device Is Feasible and Clinically Consistent, Critical Care Medicine, 2019

Hi
Below are some thoughts I had generated for another venue, but I think are relevant here. My understanding is current version is machine set/delivered breaths only (ie not sensing), which is problematic.
Short version is that forced breath-only machine is not a good solution, on multiple levels. It is much harder to maintain vent synchrony (because when the patient wants to pull a breath, they can’t), requires higher levels of sedation, and in turn generally means longer times on the vent. As an aside, this mode is called CMV or controlled (or continuous) minute ventilation.
The ventilation we usually use is AC/VC – this is assist control/volume control. It provides the breath at a backup rate, and if the patient wants to breath over, they can trigger then vent to give a breath. That is an “Assisted Breath” This is the mode we really need for patients with ARDS
Plenty of other thoughts, but for sake of staying on point will keep it to these for now. On a side not though, once patients are breathing spontaneously, ambu-bags are pretty problematic.
Thanks for your feedback. We do not think this ventilator would replace conventional ICU ventilators. When all conventional ventilators are being used in a hospital and there’s a critical shortage of mechanical ventilators, we think this ventilator could come into play and provide mechanical ventilatory support to COVID-19 patients until a conventional mechanical ventilator becomes available.
The control team is working on using the pressure sensor to implement AC/VC mode. Once successfully implemented, the team will share the methods. Until then, if a hospital needs to use this ventilator due to critical shortage, we think it’d be best for the patient to be paralyzed. Once AC mode is available, we will inform everyone.
Hey Duncan,
These are all great points. I agree that the heavy level of sedation required for these designs could be problematic for patients who need AC/VC driven ventilation. My question is, could the proposed device work as a bridge device for patients who are waiting on a vent to be freed up? This may be its main mode of utility and could keep us out of the ‘Italy scenario’, especially in the field hospitals being set up by the Army Core of Engineers across the country. I don’t have a clinical background as you do so any feedback on the idea is appreciated!
How can I upload a file? We have contacted our Congressman and have received the recent release from the FDA which outlines what is allowed/required to allow the development of a ventilator.
From what I have read online, pressure control seems to be the way to ventilate patients with ARDS due to the changing lung compliance. This way, tidal volumes do not need to be adjusted over time. Have you considered pressure control? I imagine that this might be harder to implement using the current architecture, though.
Discussion includes a mental picture of a Y connection of Inspiratory and Expiratory flow lines at connection to ET/Trach with a Proper Valve Mechanism to minimize dead space rebreathing of CO2. My suggestion is a transition to a coaxial (3D printable) joining of inspiratory and expiratory tubing, such device close to or even a part of ET/trach. One could even place a flapper valve (small version of those used in Mine Safety Administration approved respirators) at the end of the inner (inspiratory) coaxial feed, although the positive pressure of the feed should make this valve unnecessary. This suggestion is essentially that a coaxial joint (equal flow restriction in/out driving dimensions-diameters bases on cross sectional areas and lengths until transition to larger diameter feeds) rather than a Y joint will allow minimization of CO2 rebreath volume.
I agree the use of a ventilator would be extremely limited without breath detection and assist control capabilities.
Still, for my purposes, in its current inception, this is still useful device with a limited and defined role. I work with a group in Afghanistan, which is extremely, extremely, poorly resources. (They cannot get Raspberry-Pi or Arduino boards, or even pressure sensors and spirometers.) But the country itself has a total of all but 300 so-called “ventilators,” some of these actually being O.R. anesthesia machines. (I assume from your post that you’re an anesthesiologist and know how limited those are.)
The plan for this device would be as emergency ventilators that patients could be placed on for a limited amount of time while they wait to be rotated onto one of the properly deployed 150-200 or so “full service ventilators.”
Either way the situation is looking extremely dire for this country of 38 million people with very poor public health and medical infrastructure. Their solution is really going to be far, far more upstream in terms of physical distancing, PPE, bona fide testing, contract tracing, etc. But again, their public health infrastructure is quite limited….
Breath detection and assist control appears to be in the works. Awaiting word on the code teams are working on for the Arduino and Raspbery-pi boards. Once they have that, it will be a totally different ventilator with far greater utility and deployability.
Survival rate of covid 19 patients needing intubation and ventilation is only 20-40%. Also, some patients remain mentally compensated with relatively normal BP and heart rate despite very low oxygen levels. Some doctors are questioning whether the high peep ventilator treatment approach may be causing pressure damage to the lung; and questioning whether covid patients are experiencing “high altitude” sickness in addition to, or instead of ARDS. Because of this, some doctors have advocated using high flow nasal oxygen and CPAP before reaching for intubation and ventilation. A problem with CPAP machines is that they exhaust and aerosolize unfiltered viral containing patient expired air into the environment, potentially endangering medical support staff. I have posted a you tube video showing how to modify a CPAP machines to hepa filter patient expired air. If interested, go to you tube, and place my name “larrygessman” into the search engine to view.
If you see my repo on ventilators: https://github.com/hesingh/ventilator
Find this link: https://www.statnews.com/2020/04/08/doctors-say-ventilators-overused-for-covid-19/
Also, at the repo, you can see how others have modified CPAP machines.
I agree with Duncan on the problem of using any kind of mechanical Ambu-bag in a patient who is starting to breath spontaneously. The transition from a fully ventilated patient to one who needs support and finally weaning will be very difficult using anything too simple. Patients will fight the vent, not get good tidal values and may even get negative pressure edema or worsen their condition.
Please note this, also: COVID patients get ARDS (Acute Respiratory Distress Syndrome.) This is the same problem we see in many sick patients (severe sepsis, SARS – Severe Acute Respiratory Distress – and others). ARDS is a generic term for when a lung fails due to an inflammatory process. We have no antivirals. The only treatment for ARDS is to be kind to the lung parenchyma and let it recover. A patient who is pulling their own breath, with a bit of support, is physiologically in a far better place that a pure positive-pressure machine breath.
Thank you for your input. Please see reply to comment above.
Hey Ben,
I agree 100% with your concerns. Would it be possible to move a patient to a modified CPAP per the link below as a bridge device once the patient is able to partially breath on their own? I’m working with a CPAP manufacturer that is retooling their lines to aid in the crisis so any feedback on this option is welcome.
https://www.ems1.com/ems-products/medical-equipment/airway-management/articles/airway-management-adjustments-in-the-era-of-covid-19-0RrHWNl1MpLw95dY/
I am working with a multidiciplinary team in Peru to design anf fabricate a similar system. We have two questions:
1- Have you thought in any way to measure the % of O2 that you are giving to a patient? We have been talking with hour Ministry of Health in order to validate the characteristics we are proposing and % of O2 is somehow critial for patients with ARDS. We are still solving this issue.
2- In the pictures we have seen until now, the expiratory valve is part of the ambu, after that you connect a tube that goes to the HME filter and then to the patient. During exhalation, have you considered the volume that will be inside the tube when an inspiration starts? In essense, the amount of CO2 that would go inside the patient again? Because of this we are proposing two solutions, the first one would be moving the sensors and the expiratory valve near the patient, the other one would be using different channels for inspiration and expiration with a Y connector / valve, but that would increase costs beacuse we would need two sensors for each one we had.
Thank you for your questions and comments.
1 – There are medical air-oxygen blenders one can use to set FiO2 and titrate the amount of oxygen with better control. A less elegant way may be to lower the O2 flow into the manual resuscitator and if lowered below the patient’s minute ventilation, the manual resuscitator will then draw in room air and thus delivering a mixture of room air and oxygen that is difficult to trend what the exact ratio is. In ARDS, this becomes relevant because ARDSnet mechanical ventilation protocol’s PaO2 goal is 55-80mmHg. (http://www.ardsnet.org/files/ventilator_protocol_2008-07.pdf) It is feasible to achieve this without an O2 sensor. The engineering team is exploring O2 measurement as a safety feature currently.
2 – We have onboarded clinicians to guide our efforts since the first prototype was built. The team has addressed the problems with the breathing circuit and a newer prototype has been built with clinician input. Information about the updated design will be released soon.
Thank you for your reply. We have though of having a flow meter in the oxigen intake to try to estimate the blend inside the self inflating bag using the difference in volume before an inspiration. Regarding the two ideas you mention:
1- Medical air-oxygen blenders would work, we would need to close the air intake of the ambu bag and continously use the oxigen intake (would be the air-oxigen blender intake).
2- As you mentioned, it would be difficult to measure the exact blend, and for ARDS patients they tend to work with those values.
I will be checking the update as regularly as possible. Thanks!
We are aiming to provide these emergency ventilators at a mass level as critical care ventilators are shortened in Pakistan.
Here are some queries regarding the Gas Pathways:
• Can we use an HME filter instead of HEPA filter?
• Does MIT Ventilator’s gas pathway follow the Biocompatibility standards for the breathing Gas Pathways (ISO-18562)
• Does MIT Ventilator’s gas pathways follow Biological Evaluation Standards (ISO-10993)
• Should we go for the above-mentioned tests (listed in the above-mentioned standards) before deploying the ventilator into the hospitals?
• Should we use a single breathing circuit as it is shown in the MIT vent or should we go for a double limb breathing circuit (which has a Y connector for inspiration and expiration)
• The maximum time for the usage of this ventilator has been directed for up to 6 hours according to link given above, but can we prolong the usage as we are following the electrical safety standards (ISO-60601) in the making of this device?
Note: In our Vent design, we have also used an ISO-10993 compliance Flow sensor by Siargo which also tells the pressure and temperature of the gas flow and all the other components used in the making of the breathing gas pathways are medical grade.
We need help as hospitals are out of ventilators and want to develop this vent as fast as we can.
I’d be waiting for a quick reply.
This is a super good concern. We have looked into it in two ways: first we have tried using a very short tube between the bag and lung, in order to reduce dead-space air. The other method would be to use a separate inlet-outlet breathing circuit, so that exhaust never enters the source tube. Thanks!
Yeah! Those are the two ways we are considering, in fact the first one is our approach, but you end up with flow and pressure sensors after between the expiration valve and the HEPA filter, this would be around 15 cm long, so that would be the volume that would get recycled.
On the other hand, the other would be use separated inlet-outlet circuit using a Y valve, but in that case you need to double the sensors, that affects the costs of the device. At least that is how we picture it roght now.
Hi all, my understanding from the comments mentioned above is that the ventilator used for ARDS assists patient’s breathing and requires flow sensing, which is not included in this device. Am I correct?
Thank you for your question.
Triggering of assisted breaths can be achieved with flow sensing or pressure sensing. Our prototype has pressure-sensing capability and the control team is working towards implementing AC/VC mode.
In ARDS, patients are always mild or moderately sedated and often get paralyzed during mechanical ventilation. Those who are not paralyzed should have the ability to get assisted breaths. However, if someone is paralyzed, they cannot trigger assisted breaths so mandatory volume control or pressure control mode of ventilation is used.
Thanks for working on this. I imagine using this device as a way to free up what otherwise would have been a set of hands. Which may be very important as the SOCCA has predicted we may run out of ICU docs before vents. Manpower may be an under-appreciated resource in the coming weeks.
I ventilate patients all day in the OR and I make the transition from VCV and PCV to PSV to spontaneous modes on every patient. I also transport patients to the ICU from the OR on Ambu-bags while the patients are either breathing spontaneously or not. It’s possible to bag someone for an indefinite amount of time but’s not ideal as we all know. You can bag someone who is breathing on their own or you can assist them. It’s a little tricky with an Ambu-bag to assist someone as the bag is not as compliant as the bag on an anesthesia machine. So it’s a little harder to feel the patient initiate a breath than with the soft compliant anesthesia bag.
I like your design. You don’t have to worry too much about dead space and rebreathing CO2 since you are using an inspiratory and expiratory limb and the only dead space if what is found past the y-connector assuming you have functioning one-way valves. You need flow (and thus volume) sensors in both limbs; a pressure sensor, one-way valve and FiO2 in the inspiratory limb; a HEPA filter and either a one-way valve or an adjustable vacuum waste gas system on the expiratory limb; and of course the electronics and controls to run the thing. You can use the existing PEEP valve with the kit and you can use an oxygen:air blender commonly found in places like the NICU to control FiO2.
Of course PCV would be the easiest to do and yes it would require a deeply sedated or paralyzed (chemically) patient. This would be somewhat of a bridge to a conventional vent. The next step would be to incorporate PSV to the vent design. There are a multitude of settings we use to control how the vent senses and delivers a breath to a patient. For simplicity sake, I think the two most important are the flow trigger (at what flow does the vent sense a breath and deliver assistance) and trigger window (what time during the respiratory cycle will the vent respond this way).
If it is possible to provide real time pressure measurements, we can see when auto-peeping happens and if we can see inspiratory and expiratory flows we can see breath staking. This will help us manipulate pressure, rate, I:E in PCV.
Do you have a sense for the percentage of patients that would be sedated in current (or near-future) circumstances? Is the number of patients great enough that having a preliminary model for sedated patients only would be worth it? It would free up a hospital ventilator designed for SIMV/PCV/PSV.
Andrew, all patients will likely be sedated to some extent while on a ventilator. So I would say 90-100%. It is possible to have awake patients, intubated on either a control mode or an assist mode but this is rare and it typically only happens on lower vent settings in healthier patients. Not the ones who are declining clinically. The problem is that deeper sedation is usually required to increase ventilator synchrony. In other words, we need more sedation for a basic ventilator to work with that patient. Problem is that we have been warned to expect shortages in sedation medications as well and to conserve ahead of time.
Yes, the number of patients is expected to be (or is already) high enough that having a ventilator with those basic modes would be useful in certain situations. It would be possible to move patients from a basic vent to a more advanced vent as clinical situations evolve.
Dear Scott,
Thank you for your insights and comments. I agree human resources will be at a critical shortage as well as many other things like ventilators and PPE that everyone is talking about.
You are correct that the MIT E-Vent does not have all the whistles and bells of an ICU ventilator or anesthesia machine. We are aiming to provide design specifications on the minimum core requirements to be able to oxygenate and ventilate a patient with ARDS safely while trying to abide by the ARDSnet protocol as closely as we can.
Once we implement AC mode using an already existing pressure sensor in our design, the MIT E-Vent will automatically trigger a breath when the patient attempts to take a breath. When the updated prototype information is released, we would appreciate more feedback.
Thanks for the work you are doing!
From what I have read online, pressure control seems to be the way to ventilate patients with ARDS due to the changing lung compliance. This way, tidal volumes do not need to be adjusted over time. Have you considered using pressure control? I imagine that this might be harder to implement using the current architecture, though.
Hi all, super nice effort! I have a question: Do you plan on manufacturing this at MIT or do you plan to partner with another company for production? I am a graduate student at MIT EECS and I can help you with the design and manufacturing if needed.
i would like to get a manufacturing hub going here in humboldt county ca, goodluck to all and thanks on behalf of all for your willingness to help. do you have an idea of what kind of machines and facility would be needed to start building? do you have a dream set of staff? maybe a programmer, a couple engineers, a few techs, support staff, ; any interest in working in northern california, or working together? nonprofit trying to organize around this idea, thanks Stew 707-714-4205
Hello Emre
I am Lakshmipathy from India, we have small manufacturing set up in South India , we would like to support the community in developing this ventilator, kindly advise how to proceed
Email id : pathy.msl@gmail.com
Regarding the key ventilation specs, PEEP should be 5-20. There is a high-PEEP arm which we use sometimes although rarely. And the mechanical valve in the Ambu-bag set goes to 20 anyway. Allow inverse ratio ventilation with the I:E ratio. This is particularly helpful with very stiff, non-compliant lungs like what we typically see with ARDS patients. Otherwise, looks great.
We will update the recommended specs on PEEP to 5-20 cmH2O. Thank you for the feedback.
For very stiff, non-compliant lungs where inverse ratio ventilation is required, we would suggest that clinicians free up a conventional mechanical ventilator that can do that and use MIT E-Vent on a patient that don’t need such advanced settings. There has to be some degree of resource allocation strategy where conventional mechanical ventilators are used for the sicker patients as patients weaning off mechanical ventilators. For the rest of the patients who have reasonable compliance and just need more time, MIT E-Vent may be able to fill the gap in need until a conventional ventilator becomes available. Please think of MIT E-Vent as a bridging solution to the real mechanical ventilator.
im from wuhan
i should point out that,the Ventilator should be able to connect to External oxygen sources !
Hello, thank you for joining the community and providing feedback. I’m sure there is so much we could learn from your experiences in Wuhan. Please feel free to share any thoughts you have about this device and where you may find this most helpful in its deployment. The manual resuscitators generally should have ability to connect to external oxygen sources. For those who want to control FiO2, a medical air-oxygen blender should be used off the wall supply of fresh gas and blended before it’s connected into the manual resuscitator.
Sorry, I had been very harsh on some young minds.
Your design will work. The ARDS patient can be taught to breath in sync with the machine and patients would learn fast I am sure. But please don’t sedate or paralyse the patient.
R
Thanks for your feedback. Just curious about your general practice for intubated patients… Do you keep patients fully awake and not offer any sedatives while intubated? Our clinicians mentioned awake intubations are difficult to tolerate and very traumatizing to patients.
The reason for recommending paralysis in the current iteration is because the AC ventilation mode is still in development. When we have AC ventilation implemented, we can cut back on our recommendations to just sedation.
This device is not meant to be used for non-invasive mechanical ventilation such as BiPAP or CPAP.
Well, have kept patients (rat poison cases) days together on Ambo bag.
They were not intubated and that was not necessary.
Patients closest relative was doing the pumping and they know if the patient is going to stupor or a sound sleep. An if the mask slips or the relative slows down the patient immediately awakes and shows his displeasure. This feedback was vital in our treatment.
On the worst end there were patients who go into stupor and coma in spite of our best ventilation. This was attributed to non-hypoxemic brain damage. The chief would come and take a decision on such patients. Until then we had to maintain the patient with external mask with ambu bar or a ventilator.
That said:
Experience from Wuhan should make us rethink the approach to treating ARDS.
The figures: 80,000 patients diagnosed with COVID in China
16,000 (20%) were treated on Hiflow O2 and Bi-level Airway pressure ventilation
2500 (3.2%) were intubated and received invasive ventilation
2870 patients expired
One analysis on a very limited patient sample from Wuhan says mortality rate with invasive ventilation was 86%.
But a RCT prior to COVID-19 onslaught said Extrocropreal Membrane Oxygenation did not offer any benefit and Invasive and paralyzed patient ventilation offered 40% mortality on ARDS.
The very basic concept of offering invasive ventilation for ARDS is based on not-so-sound hypothesis (increased alveolar pressure decreases oxygen debt) and further supported by some RCT.
The massive experience from Wuhan and Europe may make us rethink the role of these sophisticated machines in treating ARDS.
I think the modality of treatment (ECMO vs. invasive vs. noninvasive ventilation) in providing respiratory support in COVID-19 positive patients is also a surrogate measure of severity of the disease and it makes sense that mortality is higher in patients who needed more intensive intervention. Therefore, you cannot make your conclusions on the mortality rate statistics as you are introducing the severity of illness bias into your conclusion.
Clearly, if patients are doing well on supplemental oxygen and non-invasive ventilation, clinicians would certainly maintain them on that mode of respiratory support. One should also be vigilant of worsening oxygenation or ventilation efforts as timely initiation of invasive mechanical ventilation is important to prevent patient self-inflicted lung injury if the patient is working too hard to breathe. (See https://journal.chestnet.org/article/S0012-3692(19)32648-0/fulltext)
Obviously, we cannot ethically perform (and intellectually don’t need) an RCT to prove to ourselves patients in hypoxic or hypercarbic respiratory failure need invasive ventilatory support to survive. It is similar to not needing to perform an RCT to test the life-saving function of parachutes when jumping off of planes. (See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC300808/)
Ravi, please don’t try to convince the rest of the world that intubating patients in respiratory distress is a bad idea and that we should instead mask them all by hand by a family member. That’s insanity. According to your stated data, 86% of ETT’d patients die but you don’t mention the death rate without ETT which is likely 100% Nor do you mention the rate of VAP associate with you BMVing your patients by family members which is likely not zero. If we BiPAP patients, they spew COVID into the room and infect everyone. The 2018 EOLIA trial had significant crossover for the VV ECMO arm and as such did not prove anything as you inaccurately claim. Sorry, but as a doctor, your personal experience does not account to medical reasoning. There is no good data to say we should mask ventilate a patient versus intubate them. With all due respect, please approach this project with science and reason, not personal opinion. Lets create a low-cost ventilator to free up hands for other life-saving tasks not discount the idea of intubating sick patients.
Hi all.
Suggestion – limit your design parameters as follows, and have separate discussions on each:
1) Managing ‘simple’ ventilation for COVID 19 – these patients are just not able to keep air going in and out with adequate gas exchange and get ‘tired’. This is similar to the way pediatric patients die of respiratory failure.
2) After #1 is fixed – ARDS is a whole other animal, but there are publications on successful strategies.
3) While I understand it is not presently recommended, NIPPV can help some patients on the ‘verge’. https://www.ncbi.nlm.nih.gov/pubmed/32205957 but has the down side of aerosolizing the virus all over the place…
Ravi, please, not the CDC or WHO or any other established organization recommends NIPPV so please stop mentioning it. This project is designed to create a ventilator. If you don’t agree with that approach, please continue to have the patient’s family bag the patient but don’t spread misinformation and personal opinions not based on science.
For thread #1 – I’m thinking that if you put a pressure sensor on the output of the Ambubag, you could use it as a negative feedback on the speed of the compressing arm. It is important not to ‘jam’ the air in too fast/hard. The top left image suggests to me that if you used a ‘sine wave’ as the actuator’s default velocity curve you would most naturally match normal inspiration. You’ll have to blend that with the output of the ambubag so that the net air volume delivered matches the breathing in portion.
For thread #2
https://www.thoracic.org/statements/resources/cc/ards-guidelines.pdf – what I take from this is that you do need to be able to produce PEEP – which could be managed with a second pressure relief valve on the far side of the endotracheal tube as long as you have a solenoid that switches the air flow from the ambubag on inspiration and to the exhaust on expiration. when in the exhaust position, the pressure relief valve would be set to the desired level of PEEP. This solenoid should be close to the patient’s mouth/ET tube in order to not create more dead space in the tubing which reduces ventilation efficiency.
Werecommendthat adult patients with ARDS receive mechanical ventilation with strategies that limit tidal volumes (4–8 ml/kg PBW) and
inspiratory pressures (plateau pressure,30 cm H2O) (strong recommendation, moderate confidence in effect estimates). Justification and implementation considerations. Although our primary analysis showed no significant difference in mortality, the boundary of the CI consistent with the largestplausibleeffect (29) suggests that LTV might reduce the relative risk of death by as much as 30%. Furthermore, secondary analyses that included meta-regression and a sensitivity analysis including all trials (nine studies, 1,629 patients) supported a clinically important benefit to LTV. The meta-regression of tidal volume gradient between experimental and control groups in each RCT versus mortality confirmed a dose–response relationship to the effect of LTVs (30, 31). The initial tidal volume should be set at 6 ml/kg PBW and can be increased up to 8 ml/kg PBW if the patient is double triggering or if inspiratory airway pressure decreases below PEEP (25). The strong recommendation for LTVs therefore comes from moderate confidence in the magnitude of effects on highly valued outcomes (e.g., mortality), supplemented by our secondary analyses, and moderate confidence that undesirable outcomes are modest and their avoidance is not highly valued.
The plateau pressure would be read off of the sensor just outside the ambubag, and when it hits 30mmhg would cause the arm compressing the bag to slow or stop until the pressure went below that. If the ambubag arm could actually go ‘backwards’ a little when the pressure goes over 30mmHg you could help the patient feel better if they cough or start to ‘buck the vent’. Perhaps this could be accomplished with an adjustable ‘shock absorber’ on the arm compressing the bag.
You don’t know that the lack of ventilators was the only reason they were turned away. Thus this is not even close to a randomized controlled trial. It’s an observation. They could have been turned away because they weren’t that sick… Sorry, but you need to be excluded from this site based on a lack of understanding of basic research understanding.
We’re working on a VC version at the moment in NYC. With respect to Tidal Flow – has anyone considered running the Ambu bag through a spirometer (in reverse) to obtain an accurate / calibratable measure of tidal flow? We compress our bag via a gear attached to a cam so it’s difficult to obtain an accurate reading of how much of the bag we’ve compressed due to deformation.
We’re trying to get to an accurate Tidal Volume. Currently we’re working with different gear sizes and controlling for the distance of the CAM from the bag. In this way we can also provide multiple configurations depending on what size of Ambu bag is available / on hand.
We will publish our benchtop testing setup which we also used in pig lab testing soon.
How soon before you can do human trials?
We are getting closer to human trials as soon as we are happy with all safety features.
Seguramente este proyecto saldra adelante una muy buena forma de ayudar a la humanidad ,dentro de las consideraciones generales he visto que aun no se tiene en cuenta un sistema de alimentacion ininterrumpida (UPS) seria de gran ayuda integrarlo a todo el sistema ya que podria darse la eventualidad de fallo energetico.
En cuanto a los sensores por experiencia los sensores de ese tipo fallan con regularidad opino que debería colocarse un sensor capacitivo de proximidad son mas confiables ,a diferencia del sensor de proximidad inductivo, el capacitivo es capaz de detectar también materiales no férricos a una distancia de hasta 10mm.
Hi Everyone,
First of all, thank you so much for sharing such valuable information; it really helps. I have a query which some may find senseless but I was thinking of it and so I felt it is better to ask you guys who have a better understanding of the ventilator system.
It is the brain that gives the command to initiate the respiratory and expiratory cycle. Is it possible to sedate only that part of the brain which is responsible for the respiratory cycle so that this machine can be applied to active and passive patients both?
Hello, great question. You are asking a question about drug delivery in essence. Intravenous anesthetics and sedatives pretty much go everywhere the blood courses through and bind the drug’s target receptor. Some classes of sedatives or anesthetics can suppress respiratory drive more than others, but would require higher doses than mild to moderate sedation. The best solution would be to implement assist control mode of ventilation. If the patient has difficulty becoming synchronous with the ventilator on mandatory volume control mode despite attempts to adjust the settings, I think the safest thing currently is to either go find a full feature ICU ventilator for this patient and give the MIT E-vent system to another patient who can tolerate it better OR paralyze the patient with a muscle relaxant.
Thanks for giving opportunity to understand the criticality in this development and the efforts the team put in trying times.
I have one clarification, The patient is have breathing difficulties and not required sedation, in that time is this system support ?. Is this possible in assist control mode.
How the assist control will work,
Is the system changed from volume/ Time cycling mode to pressure cycling mode.
Is this possible to have the machine learning algorithems in assist control ( based previous three or four cycles pressure feedback auto adjust the bpm, I/E ratio With in prescribed range)
Hello, there is assist control mode that has been developed and successfully tested on a pig. However, we are still refining the algorithm right now. We will publish the code when its ready.
Will you be so kind to update this page with the information from the “plumbing” page? Especially the list of recommended hardware to address the dead space. It is very important so we can convey the information properly to the physician and staff directed to use this option.
Thanks!
I am an experienced ventilator designer, and also an MIT graduate. I implore you to not employ animal testing to test your device. It is not required nor necessary for testing ventilator equipment. None of the ventilators I have designed in the past require testing on animals. There are plenty of good lung simulators available which are excellent devices to use for testing. Nearly every ventilator company in the world uses lung simulators to verify and validate their products. Look at lung simulators from Michigan Instruments, Ingmar Medical, and others.
Imagine yourself as a helpless and terrified pig, having a tube forced down your throat and hooked up to a breathing machine. Animal testing is not only incredibly inhumane, but it is completely unnecessary. Furthermore, the results often do not correlate to the way the device performs on humans. Please show some compassion and common sense and forego the animal studies. Thank you.
Mohan
I agree with M Gurunathan’s comments. If this virus is teaching a lesson to humanity, that is all nations are equal and humanity is one. My nation can not be safe from this virus as long as some other nation is not safe. We have to go one step further and say all life is equal. In the words of Old Major (Animal Farm) “All Animals are equal” (Man being one of them). If we do not pass this simple test then this virus may be the one which will bring true Old Major’s words — “Tyrant Man shall be o’erthrown”
Controlling the inspired concentration of oxygen is important, but getting a blender to control or quantify this may not be easy. I propose the following idea. The target oxygen saturation for the patient will be 88-90%. The function of the reservoir bag in the AMBU set up is to provide 100% oxygen. One should remove the reservoir bag and adjust the supplementary oxygen flow to achieve the target oxygen concentration. Perfection may not be possible in situations like we are facing.
Dear MIT E-Vent Team: We are trying to reach out to you, to offer our (bonafide) support, either from a design stand point or from a regulatory (FDA Emergency Approval) condition. An email was sent yesterday to e-vent.mit.edu. Please reply ASAP so that we can decide in our effort.
Respectfully,
G. Alvarez
AutoPak Engineering Corp
I am only a mechanical engineer, not a ventilator engineer but I am very happy that a simplification school of thought is in session. May it prosper!!
My sister contracted Respiratory Polio in the 50s. She survived for 35 years with a very productive life as the Editor of the Toomyvill Gazette, with only 7 percent of her breathing capacity. During the daytime (awake) she always used a semi-conscious method of breathing , called “Frog Breathing” (at night it was a Rocking Bed.)
Frog Breathing consists of pulling up your sternum with your neck muscles. Obviously you have to do this with a conscious command. (Over the years it became almost sub-conscious.) Many of our Covid patients are not conscious or cogent and an instantaneous, mechanical means is necessary.
What I am wondering is whether or not a device that pulls up on the sternum could substitute for this laboriously complicated pneumatic method being pursued by your folks. Or, whether the phenominum of a Rocking Bed could be applied with less drama. (It is well to note that the rocking was timed by the speed of the A/C motor, This was a very crude but provided a mind-free sleep for her all those years.)
She died of a broken-hip/brain-anurism following a fall, not exficiation.
I am Octavio Monroy from Mexico City. I am a Rotarian.
I have a small workshop where I can make the respirator so I can help my community.
Where can I get the blueprints for making the respirator, obviously with your permission and guidance.
Sincerely,
Octavio Monroy
juocmome@outlook.com
Thanks
Please, is very important know, what model of pressure differential sensor did you use?
Has Postural Draining (lung draining by gravity) been considered as an aid for draining liquid out of the lungs?
In prone positioning, patients are placed on their stomachs for at least 12 hours a day. Prone positioning of a patient is a way to recruit alveoli, improve oxygenation, and increase lung volume. The effects are attributable to changes in pulmonary pressure distribution, rather than increased drainage out of the lungs. The current design of the E-vent is limited as an emergency ventilator without the capability to ventilate patients in the prone position. Patients who are in severe enough condition to require such positioning would likely benefit from a full feature ICU ventilator.
Hello,
My name is Muhammad Umais I have done my electronics engineering recently. I wanted to inquire the following questions which after a deep research regarding ventilators (using resuscitators), I have obtained. I hope some of you could answer my queries.
We are in a process of developing an emergency ventilator on the same specifications and designs open sourced by MIT. Our country is a developing country and to fight COVID-19, our college has initiated to produce cheap ventilators for all the underprivileged areas of our country.
Here are a list of questions which I along with my team inquire.
• What is the duration of use of this emergency ventilator (I read emergency resuscitator devices can be used for a maximum of 20 minutes)?
• Can an Ambu bag ventilator be used for all age groups?
• What are the bio-compatibility tests (exact tests) for this ventilator device (10993 and 18562)?
I would be thankful to anyone who could guide me through these queries.