Updated 1 May 2020
This page describes critical design requirements of the patient breathing circuit. This details a key dead space issue, which if not addressed, will result in a patient breathing in expelled CO2 and deoxygenation fast with immediate adverse result. For a detailed primer on Breathing Circuits read Mapleson’s Breathing Systems.
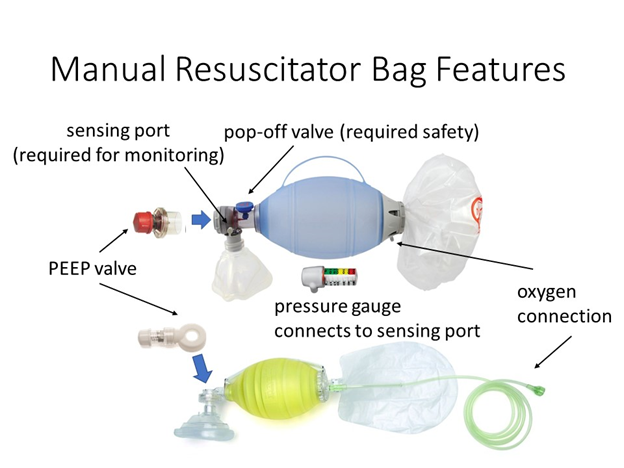
Normally, self-inflating manual resuscitators are directly connected to the patient’s endotracheal tube adapter. Manual resuscitators have a “patient valve” that directs oxygen/air gas mixture into the patient and shunts the exhaled gas out to the environment. Integrated into the end bag valve mask (BVM) are a number of critical features:
- Oxygen connection and reservoir
- Pop-off valve for safety (location not important)
- One-way valve that guides air to the patient
- Exhalation valve (this stays closed while there is any pressure on the bag)
- PEEP valve that is installed post the exhalation valve and maintains backpressure
- Sensing port for manometer connection (we use this for our pressure sensor connection)
Caution: Manual resuscitator bags are in no way FDA approved for use as long-term ventilation solutions.
Some considerations regarding how the patient should be connected to a manual resuscitator-based ventilator include:
- The ventilator must be placed as close to the patient as possible.
- Bag should be secured to ventilator to prevent an awake patient from pulling on it or otherwise disengaging the bag from the mechanism. This is a fault condition that should be detected by pressure sensing.
- Care must be taken to prevent rebreathing of CO2 due to long hoses. A fundamental challenge is the location of the one way and expiratory valves, which are typically directly integrated into the bag.
When a manual resuscitator is placed into an MIT Emergency Ventilator, or similar design, the system cannot be placed right up against the patient’s head. In addition, patients need to be turned intermittently for routine care and patients can thrash and move in their beds. Even when patients are paralyzed, the paralytic may wear off at times and we must consider how to keep the patient safe from inadvertent breathing circuit disconnection or extubation. Therefore, a safe method to extend the “reach” and flexibility of the manual resuscitator to a patient lying on a hospital bed is needed. If a simple tube is used to do so, it creates a critical safety concern of “dead space.”
Note: In a 1 m long tube of nominal 2 cm diameter, there is an unacceptable 314 mL dead space that the patient will breath in and out and not be oxygenated.
Dead space simply means volume in the respiratory circuit that does not participate in gas exchange in the lungs. Our natural anatomy has dead space as well. Considering gas exchange occurs at the alveoli in our lungs, every anatomical structure above it can be considered “dead space”: nasal/oral passages, pharynx, larynx, trachea, and primary / secondary / tertiary bronchi. Extending the tubing through which bidirectional flow of inhaled/exhaled gas mixture occurs only increases dead space.
A way to move the patient valve of the manual resuscitator closer to the patient is critical in solving this issue. Standard ventilator circuits have two limbs, one for inspiration and one for expiration, so that gases can be recaptured by the ventilator. Single limb ventilator circuits with a patient valve located distally already exist on the market, but are not necessarily optimized for use with a manual resuscitator.
Note: Solving this problem requires creativity – to the best of our knowledge, no manual resuscitator manufacturer makes an approved solution and no manufacturer makes all the parts that will assemble together correctly.
Note: Leaving a analog pressure gauge connected to the bag’s unused sensing port (not on the end of the breathing circuit) provides a visual backup to quickly inspect system operation.
Industry Notes
In reviewing products available on the market, we have some notes:
- No bag makers supply extension hoses with the appropriate fittings.
- Bags designed for reuse, i.e. autoclavable, are the only bags that can potentially survive under repeated use. We do not have any information about lifespan.
- Some Ambu bags do not have detachable heads, but they do incorporate pop-off and easily attached PEEP valves in their designs. They can only be used if extended with a separate head and extension tube. Ambu Mark 5 and Silicon Oval heads are detachable and may be available as parts. They have easily combined manometers and PEEP valves.
- Laerdal bags do have detachable heads, however in the adult sizes these heads do NOT come with pop-off valves; these are available in the Pediatric model. The pediatric model head will probably fit the adult bag.
- When a long tube is used, without a dual limb circuit and one way valving to address the dead space issue, this may affect the volume delivered to the patient; it may be necessary to increase the inspired volume.
- Addition of the HEPA filter will cause a pressure drop and may affect PEEP settings.
- Tightness of all connections is important.
Caution: In the worst-case scenario, placing the head as close as possible to the patient will reduce the dead space, but it is not an optimal or safe solution, especially for patients with reduced inspiratory volume.
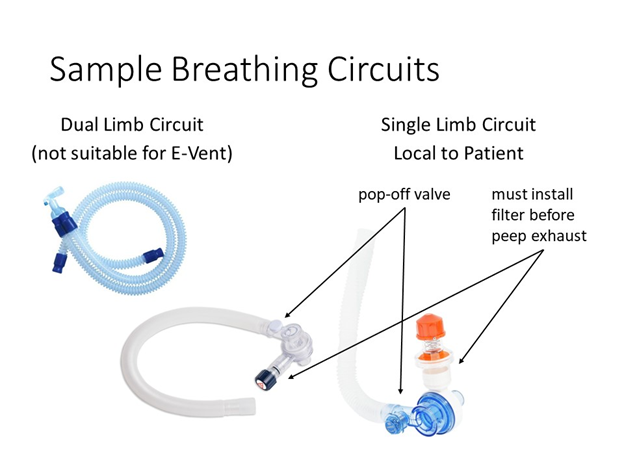
Sample Circuits
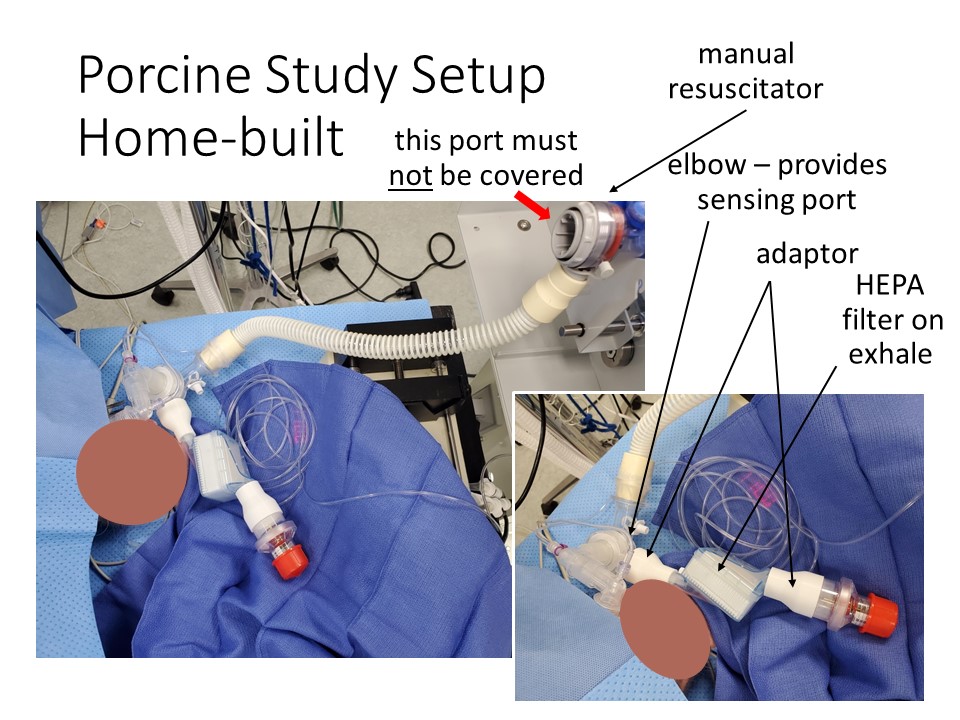
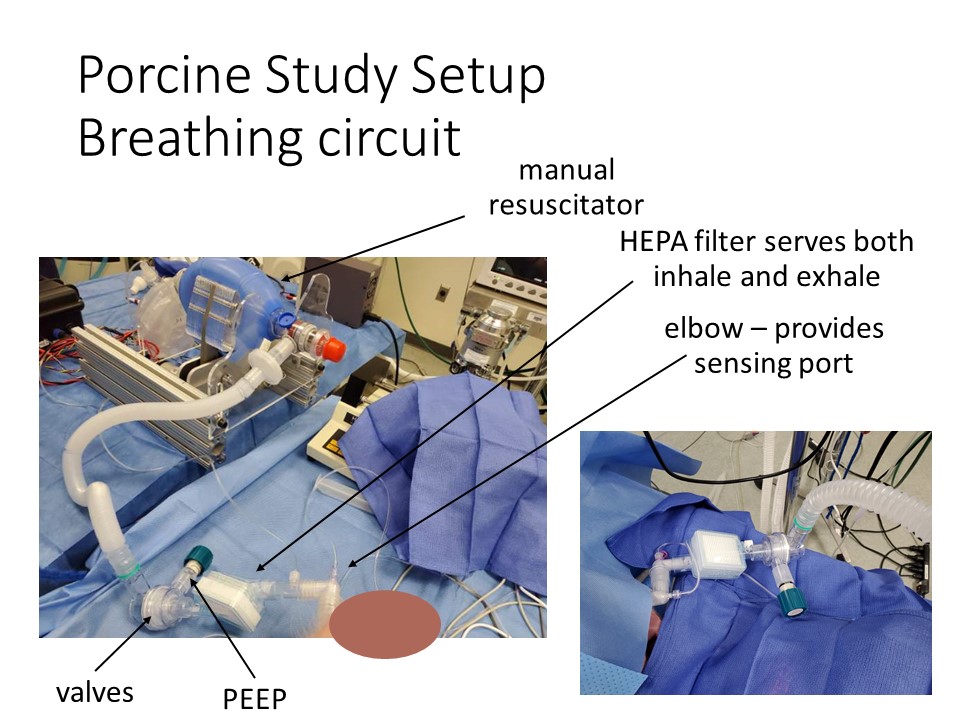
Two versions of single limb circuits are shown below. The first uses readily available components and two printed adaptors to make them fit together, with the HEPA filter placed between the exhalation port and the PEEP. The second uses a single limb breathing circuit, with most of the necessary features integrated, and a HEPA filter added inline between the porcine and the breathing circuit. This is a better position as it filters air heading both in and out of the patient, including any air that escapes from the pop-off valve. It may also help to moisten air inbound to the patient.
Note: The best location, if you have only one filter, for it is between the endotracheal tube and the breathing circuit.
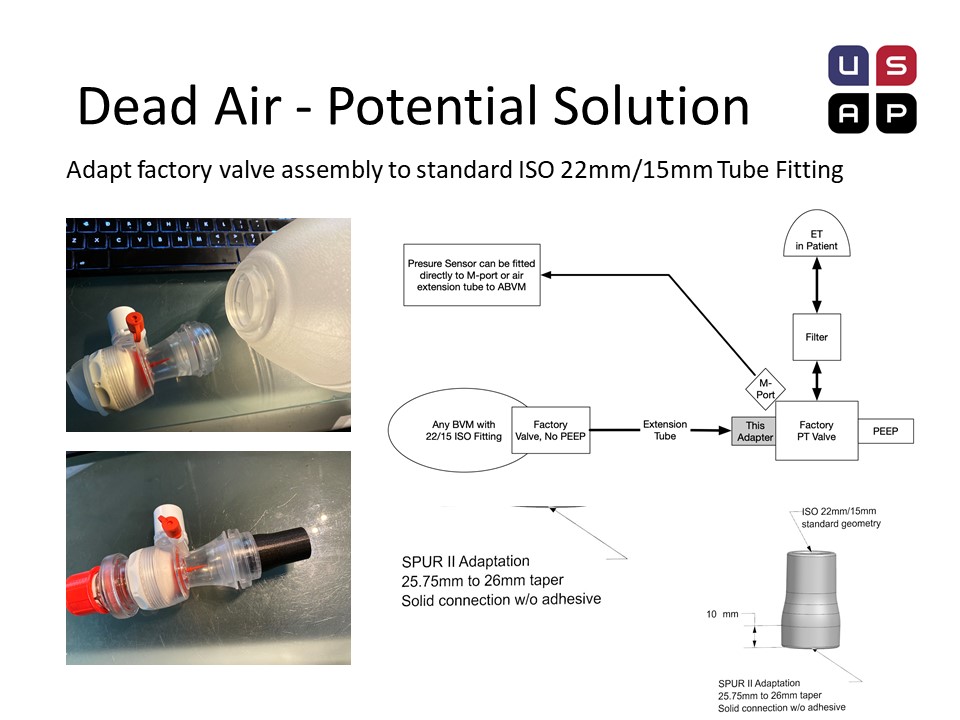
Dead Air – Additional Potential Solution
Our friends at the US Air project, an Oregon based group of fabricators currently scaling up, have dissembled Ambu bags and developed an adaptor, that demonstrates the feasibility of DIY circuits with a little feasibility.
Ambu has readily available complete patient valve assemblies, though limited information is available on their website. For example:
- Mk IV Ped (w/ pop-off and Mport) #299 000 508
- Silicon Oval (w pop-off and Mport) #470 000 503
The ideal solution is to order these valves, with a hasty solution being “harvesting” valves from disposable SPUR II units. Note: Valve is not replaceable from the bag once removed. This method places the pressure port in the correct position to monitor patient airway pressure.
Connecting these valves can be accomplished with na adaptor. All Ambu valve assemblies have a conical taper from injection molding that allows for a reliable adapter connector to be quickly fabricated. This adaptor can be 3D printed (fast, but sub-optimal), CNC milled with a sub 1 min cycle (in 6061) or injection molded (ProtoMold quote of $1.63 in 5000 volumes, in HDPE). A hose can now be used to connect this valve to an intact manual resuscitator. The extra valve assembly on the intact manual resuscitator should be inactive for practical purposes.
Caution: Care must be taken to ensure that connections are secure. Medical grade adhesive should be used.

Bingo – thanks for posting this, it is absolutely critical and unfortunately not recognized by most teams who are getting into this for the first time.
I tried to build a T with one way ventilator valves to put near the ET tube to mate with standard 22 mm tubing to solve the dead space problem. Unfortunately, the supply of these valves is scant, and/or depleted due to world wide competition for parts. I solved this problem by using inexpensive “breast pump” duckbill shaped and toilet seat shaped valves which are still available (delivered in 2 days after ordering them on amazon). The valves can mate with 1/2 inch schedule 40 PVC pipes and fittings (available at Lowes and Home Depot, or on line) and 22 mm ventilator tubing. I made a you tube video showing 1. how to make one way ventilator valves from breast pump valves, 2. how to make a T with valves to divert ambu bag compression ventilator expired air to a hepa or n 95 mask filter using the T. The toilet seat shaped valves have less airway resistance than the duckbill shaped valves, but are slightly harder to mate with pvc tubing. To view the video, go to you tube and type “larrygessman” into the you tube search engine. For any questions, email larrygessman@gmail.com
If a covid 19 patient is sick enough to need mechanical ventilation, survival rate in the US, UK, Italy and China has only been 20-35% when using full featured ventilators. Once intubated patients spend 4-11 days, and sometimes longer on ventilators. It is likely that survival will be lower in poor countries using simple ventilators. Because of this, some doctors are keeping patients on high flow oxygen via nasal cannula, and then CPAP machines for longer, trying to avoid intubation and ventilation. The problem with CPAP is that, like the MIT and other simple BVM compression ventilators, it also uses a single hose patient circuit, and exhausts and aerosolizes patient expired air unfiltered in the environment; making it dangerous for medical caregivers. I have posted a video which shows how to modify cpap to allow hepa filtering of expired air. Go to you tube and type “larrygessman” into the search engine to see how to modify CPAP machines to hepa filter patient expired air.
HME Filter?
Thanks for adding this information. Could you please check, one ventilator to be connected to multiple patient by connecting some special distributor and increasing the pumping capacity.
Vikas, that won’t work for a number of reasons, most of which are connected with the fixed repetition rate of the device. This would force all the patients to breathe at a common rate, perhaps involving over- or under-oxygenation of blood gases, would cause terror and discomfort if they are suffering from the usual ARDS-syndrome problems, and would require additional safety equipment to avoid the issues already identified in preventing lung damage (particularly in necrosed or autoinflamed tissues) through excessive pressure, volume, or overly high PEEP.
A ventilator is not the same thing as a positive-pressure air support; I recommend you look at the detail-design differences between ordinary CPAP (which is ‘not’ a preferred bridge-ventilator conversion solution) and bilevel CPAP (which is) to see more of what a positive-pressure supply solution (pulsed or not) would involve. The E-Vent design is specifically intended to work as a substitute for full invasive ‘medical ventilator’ substitution, a very different technical exercise.
I spent a long time last night trying to solve the dead space problem with longer tubing to the ventilator, to enable it to be at a slightly greater distance from the patient.
Anesthesia machine ventilators solve this problem by using two one-way valves, one on the inspiratory limb, and one on the expiratory limb of the circuit. They are built into the machines, so are at a distance from the patient’s endotracheal tube. For cases with the patient’s bed turned, we use extensions so that the machine/ventilator is quite a long distance from the patient’s endotracheal tube.
I was wondering if inexpensive one-way valves could be made (?3-D printing, or another approach) and placed just before an HME/HEPA filter on the patient’s ETT using Y-tubing, similar to an anesthesia circuit. Alternatively, this could be handled by solenoid valves timed with the ventilatory cycle, but I would think patients would need neuromuscular blockade (paralysis) to avoid fighting the ventilator. You would have to fabricate a Y-piece with the appropriate sized connectors (they are standardized) containing two one-way valves, positioned one on each limb of the Y, with the flow direction opposite each other.
I would think this could solve the dead-space issue. Please someone chime in if I am missing something here.
Another company is working on 3d printable check valves, based on the check valves in a painting respirator. It is not too hard.to make them. They don’t need to seal perfectly to reduce re-breathing.
Also, your vent should have a sigh function, if possible . (hold pressure for a while to open up the lungs)
Steve Harrington, Flometrics
Could you post the name of the company?
We are developing a non-invasive Respiratory Assist Device based on CPAP and BiPAP devices. We introduce oxygen and contain and filter all exhaled gas form normal breathing, cough or sneeze. We have developed simple check valves, with various cracking pressures, that use 3D printed housings and silicone valve elements cut from flat sheet. We are working on completing a video, after which I’ll post the video and the models on our website at http://www.novoengineering.com. Hopefully by tonight.
Yes, we are the company that Steve from Flometrics was referring to BTW.
We do have a little pause during which we take a pressure measure. Releasing pressure on the bag causes the expiration valve to open.
What about using water as the piston to pump the air? A large U shape pipe filled with water. One leg would be closed at the top with in and out check valves. This would at act as the air pump. The other leg is open at the top. A displacer (not a piston) lowered into the water on the open leg provides the compression. The delta in height between water in both legs is a defined pressure. The air volume is monitored by the change in height of the pump water column.
I’m confused.
If you use a BMV valve at the patient. you effectively have a “Y” and 2 check valves in place.
The duckbill valve will not allow patient Exhalation back into the hose from the BMV.
The other valve will not allow Inspiration from the room via the PEEP control.
How is there any dead space created?
It looks like the issue is that there is not standard plumbing enabling a hose to be attached between the bag, which can’t be too close to the patient, and the BMV valve which needs to be as close as possible to the patient.
The tubing could be longer, if it was narrower, and still be the same dead space. Could the motor/ device handle the additional pressure?
+1
It is critical to eliminate dead space, not just minimize it. If not the patient will re-breathe and de-oxygenate.
This is going to save lives especially here in africa,
we have no OPTION !!!
EXCUSE ME I’M NOT A DOCTOR OR SOMETHING LIKE IT BUT I HAVE A LOT OF INTEREST IN DOING IT IN MY COUNTRY PEOPLE DIE IN THE STREETS AND NOBODY DOES ANYTHING, I JUST WANT TO KNOW IF SOMEONE DID IT AND TELL ME THAT THIS MECHANICAL RESPIRATOR IS PLEASANT AND GRAPHIC THIS CAMILOPACHECO123123@GMAIL.COM
How do you monitor and apply the inlet pressure to the patient through Inlet Valve ?
Here is a loose thought. How about encasing the bag in a rigid container just larger than the bag itself. Then you control the pressure inside the container to actuate the bag. The bag and container could be placed just as close to the patient as normal with a pneumatic line or two leading to the control box. It would be a bit heavier.
This has been tried, but we believe that achieving safe closed loop control is harder.
N H, what is the main difficulty with achieving safe closed-loop control with the approach suggested by donald briner? Is it difficulty with controlling the exact volumetric compression of the bag? I was thinking about a similar approach, but with an outer bag rather than a rigid container (although either could work). The (small) space between the bags would be filled with water, and additional water would be introduced to squeeze the inner bag; because water is incompressible, the volume of air squeezed out of the inner bag would be almost exactly equal to the amount of water introduced. The total water volume would be constant (a closed system), but the amount of water moved in and out could be easy and accurately controlled with a variable-stroke piston. The piston could be actuated using either compressed air/oxygen or utility water (or an electric actuator, of course), regulated to appropriate pressures.
If incompressible water is the driving fluid, then position controlling the piston position is the same challenge. If a compressible fluid is used to compress the bag, then it’s better to look into a direct pressure control system that replicates many of the old electricity free vents. This presumes a pressure source, which may not be readily available in field hospitals.
N H, thanks for the response. Assuming that the purpose of position control of the piston is just to ensure than the correct volume is delivered each stroke, that could be accomplished pretty easily with adjustable hard stops for the piston, movable reed switches on the outside of the cylinder (triggered by a permanent magnet in the piston), etc. The piston can then be actuated by any pressurized fluid, such as oxygen or utility water (which typically comes in around 40 psi; since the max allowable bag pressure is about 0.5 psi, you would want a ratio of areas on each end of the piston of 80:1; this means only about 10ml of utility water would be exhausted per 800 ml breath). I assume even field hospitals, at least in the U.S., will have utility water lines run to them, although I could certainly be wrong.
In any case, this system could also be actuated with a motor, or possibly a solenoid that pushes directly on the piston. That eliminates the advantage of not having to source suitable motors, but there are other advantages as well: the sleeve could be manufactured very inexpensively and quickly by companies that make film plastic products, and it would be very compact. Also, measuring the cylinder pressure, i.e. for the purpose of sounding an alarm, gives a direct indication of the bag pressure, without having to tap the pressure sensor into the bad or airlines.
I should mention that, having looked at the various BVM designs out there, I don’t see a good way (on most of them) of sealing an outer bag or box at either end of the inner bag like I suggested before. I think a better approach would be to make the outer bag double-layered, with the two layers seal-welded together around the openings at either end. The BVM bag would just be inserted into this double-walled sleeve, and water would fill the sleeve, accomplishing pretty much the same thing as before.
It appears that you are using a reciprocal actuator to pump the bag.
I believe this design can be simplified and more reliable by using a rotary (peristaltic action) actuator.
The reason we settled on the bag is it’s ability to control volumes with some reasonable precision.
Part of the reason that the ventilator has to be at a little distance from the patient is so that if the patient turns their head (or a caregiver turns their head) the patient doesn’t become accidentally extubated (their endotracheal tube is pulled out of their airway). Trust me, this is easier to do than you think. That is a potentially lethal complication in these patients. It becomes an even more difficult problem if patient’s are proned.
There needs to be some length of flexible airway tubing between the patient’s ETT and the ventilator.
Alan, can you provide us more information on the frequency with which patients are proned? I was referred to this article (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6026253/) but don’t know how much the practice is being applied to COVID-19 ARDS patients with or without ventilation. Would one practice compete with the other? Thank you.
I don’t practice in an ICU setting (my practice is mainly CV, general, trauma, and OB anesthesia), so I don’t know the frequency that patients are proned. But with ARDS it is known to be helpful in many patients, and critical care physicians and anesthesiologists are well versed in proning patients. Anecdotally, it is being used with some frequency in COVID-19 patients on ventilators. As the article in Chest you referenced indicates, clinical trials have been difficult with ARDS patients – it’s hard to get clean data, and the results have been mixed.
To quote the paper, “prone positioning moves from a salvage therapy for refractory hypoxemia to an upfront lung-protective strategy intended to improve survival in severe ARDS. Indeed, prone positioning has never been proved to afford a survival benefit when used as a late rescue therapy for refractory hypoxemia.” I think this lesson has been learned by intensivists – hence the push for early proning in ARDS patients, and COVID-19 patients.
I’m really drifting from my specific area of expertise. Hopefully there is an intensivist on this group who can provide a more definitive answer. And I don’t know about proning patients on CPAP or humidified high flow nasal cannulas. Personally, I’ve not seen it. But these are different times we now find ourselves in.
Hello! I’m confused about calculating the volume of air we have to deliver to the lungs. If, for example, the required air volume is considered 400ml at atmospheric pressure then it is found as a volume of 200ml at 40H2O pressure (for example). Even so a normalized volume calculation could be made at atmospheric pressure (p1V1 = p2V2) but we should be able to approximate lung volume.
How close do you think you may be to a workable design (I.e. not necessarily the final design but a first version that could be revised in a second round)? The reason I ask is the COVID19 wave will begin peaking in the US in just a couple of weeks. It is possible to have teams (First Robotics team mentors and various businesses) make component parts for delivery to MIT for assembly. We would do this at likely no cost. If a workable design is produced, the production and assembly could start almost immediately and complete units could be sent to states and foreign governments as needed. Each completed unit is worth perhaps two or more lives.
I second this question. Both my company and my FIRST robotics team could contribute to production (while maintaining strict social isolation and disinfection procedures) when the time is right. Is it possible to release a preliminary bill of materials to facilitate planning and preparation?
Si se encamisara otro tubo mas pequeño dentro del que se introduce en la traquia y por medio de una segunda vejiga se extrae el CO2 y se diseñan pequeñas válvulas periféricas en las extremidades de los tubos tendriamos dos vias en una evitando el espacio muerto, espero sus comentarios
Cool stuff guys! However, I am a little confused. In the beginning, it is said that manual resuscitators are connected to ETT. Then, further down the article, you guys talked about issues concerning the use of a mask and single-limb tubing.
Does this mean that if I have dual limb tubing for ET, I shouldn’t have to worry about plumbing and dead space issues?
The dual limb is used with a machine that controls both in and out, which is needed with anesthesia gases. With the bag there is only one way flow to a patient, hence a single limb.
The ET tube is a single tube but is conducting gas flow 2 ways, in and out. The out/exhale has CO2 and should not be mixed with the next breath; that it is called rebreathing and will raise blood CO2. The typical use at the Ambu is direct connection to the ET. Just past this connector elbow is an exhale one way valve allowing the CO2 exhaled breath to escape the breathing system. Most Covid19 systems would put a HEPA filter and PEEP devices next. So if there is a need to place an extension between the Ambu exhalation valve and the ET, this would be dead space (2 way traversing gases with mixing and rebreathing). Yes, spontaneously ventilating patients can be on a high flow ‘T-piece’. Often done in the MRI scanner. But we are not talking about spontaneous ventilation; this is positive pressure (invasive by ET) ventilation. Mapleson described how to deal with dead space, see the intro to this plumbing section. Move the fresh gas or move the exhalation valve are the ways that have proven effective.
¡¡Ya tengo la concepción de las vavulas para los tubos encamisados!!
There are two ways to fix this.
You can use a dual limb or a single limb both will work.
In a dual limb you need an inspiratory and expiratory limb and a wye. What most people are forgetting is there is a continuous flush of oxygen. From the bag valve mask (bvm).
That eliminates the rebreathing on the inspiratory limb, the wye attaches to the patient via a flex tube, and the expiratory allows u to attach a filter at the end for filtration. This is usually done on almost all ventilators
The second way is to use a single limb circuit, and use a one way valve near the patients flex tube, this prevents rebreathing and a continuous flush from the oxygen
Both works co2 rebreathing is not an issue
In fact the second method is often done when patients needs to go for an mri as a ventilator is not able to enter into an mri area
The second method is also done so that when on a ventilator a patient when cuff deflated allows for speech
This method requires a t piece as well to allow for exhalation
Incorrect regarding continuous flushing. The fresh O2 is presented to a valve outside of the ambu bag. The valve only opens when there is low pressure in BVM (only during the expansion of the squeezed bag). At all other times the O2 is just mixing with room air outside of the BVM. In fact, because the expansion of the bag is SO FAST (maybe 100 l/m) a lot of room air is also entrained with the 10 l/m O2 flow near the valve. Ambu has known this problem for years; the early models couldn’t reach ~100% fiO2 because of air entrainment. That is why they attached a fresh gas reservoir bag to collect the O2. This is the clear, thin bag at the trail of the BVM device. The O2 tubing often transits this reservoir bag. Take one apart. You will see that the O2 just fills the resevoir not the BVM pressurizing/squeezing bag. Therefore the constant O2 flow does not “flush” the breathing circuit. Only a squeezed bag breath will move gas in any tubing attached to the BVM.
I have concerns about using a HEPA filter on the exhaust. We use in line HEPA filters in Pharma production but often experience problems due to the filters plugging up and creating a significant increase in backpressure.
An idea since we want a specific backpressure in inches of water is to simply use a water column. If a tube is connected to the discharge and the tube placed in a container with a predetermined height of fluid the discharge air would percolate thru the fluid. If the fluid were a disinfecting solution it could be used to control both the backpressure and decontaminate the discharged air. If the clinician wanted to adjust the backpressure, they could just adjust the fluid column height.
Hi Frederick, this is a nice idea to dip the exhaust tube in disinfecting solution or hot water, thus having adjustable water column for varying the PEEP. If required a low cracking pressure Non return valve can be implemented, if there would be any chances of fumes/steam going back to lungs. Please share if you have any review about this system by any clinician.
Team you also please suggest your .
Thanks & Regards,
Vikas Mali
What if the bag is surrounded by a self-expandable net (some lines with little springs) and inside a small case, just big enough to fit the bag. Then the torque to press the bag is exerted by a single line, similar to a bike brake. This way all mechanical-electrical gear can be as far as needed, and the bag as closest to the patient as possible reducing dead space. This way it is only needed for the case to be fixed in a way torque is correctly transmitted (speaking out of total ignorance here, but the bag could even be attached to the patient’s forehead and move with him/her).
Greetings,
For my project I mocked up setting the Fi02 using venturi plugs and a standard parts. It’s a kitchen counter mock up, so please forgive the masking tape and “plug” analog. https://photos.app.goo.gl/yPmgS7QcQayEHpox5
Good luck, Dave
Yes, a Venturi system could be used to provide control of the fiO2. Most Respiratory Departments in hospitals have controllable plastic humidifier/fiO2 controller canisters with high flow congregated tubing which can be used to fill the thin, clear ‘ fresh-gas’ reservoir bag of the Ambu system. The connection of this controlled fiO2 congregated tubing and the reservoir bag CANNOT be tight; excess fresh gas must be able to vent to the room. If you don’t vent it, the reservoir itself will pressurize and one of 3 things will happen. 1. The Venturi calibration will stop and the fiO2 increase leading to approach of 100% O2. Not deadly but not DESIRED. 2. Pressurized reservoir will open the intake one way valve and may keep it open making some of the squeezed volume to flow retrograde into the resevoir; screwing up the volume of the delivered breath. 3. Potentially pressurizing the reservoir, the squeeze bag, (opening both inflow & outflow valves of the Ambu) and pressuring the connecting tubing, ET, and lungs.
Can we apply the numerical analysis technique of
Smoothed Particle Hydrodynamics (SPH) for the efficiency verification
of this equipment as a whole?
Greetings,
I am from India, Here a lot of agencies NGO and govt organizations are working and releasing several models of ventilators, but I found MIT Event is certainly differ from all mechanical replacements, Really a clinically proven design, Hope you have a successful design now, will u release a BOM and technical details of the same as a document in this registered group of members, if so we like peoples also can develop the same as a model and contribute our suggestions for your consideration. We are an embedded systems design lab and very much interested in this concept. with your permission we would like to be a part of this project and like to work together for a better world.
availability of parts, design logic, assembly methods etc are helopful.
We have already developed a mechanical model with gear wheel mechanisms, but clinical aspects are not great with that design,
hope to hear from u the best.
Hi, I believe it is important to create a diverter on the expiratory valve of the ambubag and send it through an inline expiratory filter before releasing it into the air, SARS COV2 is airborne, and can easily contaminate the ambient environment.
or alternatively, remove the valve assembly from the ambubag(you need an ambubag which can do this) and replace it with a one way valve, use a double circuit ventilator tube with a Y tube connector, with a separate expiratory valve and filter plus PEEP valve on the expiratory side.
Is anyone here considering “dead space” as a potential source of viral re-inhalation as a source of exposure that would thus lead to positive feedback for disease (exponential) progress? It seems to me that considering adding this as a design consideration for a “viral” ventilator may be important. Ideally a design could be reached where the ventilator could assist in removal of viral discharge from the patient’s lungs and ventilator system and safe stowage for disposal.
Hello! Thank you for your question.
As long as dead space is minimized in the breathing circuit, this is not a major concern. Dead space is ventilation not involved in gas exchange. If the machine were set to only ventilate the dead space, this would have significant implications in CO2 rebreathing and potentially deliver a hypoxic gas mixture to the patient. Our circuit minimizes dead space by incorporating the PEEP valve and diverter as close to the patient as possible.
Additionally, utilizing a PEEP diverter with the proper rubber gasket will ensure that the expiratory gases are only released through the PEEP valve and not back into the extension tubing, thus allowing one-way flow. That being said, the design of the machine employs a mandatory HEPA filtration between the patient’s endotracheal tube and the remainder of the breathing circuit with an additional option for a second HEPA filter on the patient’s exhalation end to prevent aerosolization of the virus. The first mandatory filter between the endotracheal tube and breathing circuit ensures HEPA filtration of both inspired and exhaled gases. The optional second HEPA filter can be added in the exhaust limb before the PEEP valve ensuring that expiratory gases flow through a second filter prior to reaching the environment.
In regards to other sources of viral discharge, such as those removed during endotracheal suctioning, the current design imposes no constraints on traditional endotracheal suctioning practices in patients with droplet or airborne isolation precautions in place. Suctioning for the removal of airway secretions is done through the endotracheal tube itself and not the E-Vent.
hi
can i ask about ( Finger-bag max contact area: Abag = 80 mm2) how this value was calculated ? With this value the power value( 50.2 N ) is incorrect !! can you help me please
In India, a group of doctors are against using this design. They can be convinced if it has been deployed somewhere in US or other countries. Can you please let us the current status of this design on testing, clinical trials, certification and deployment at Cambridge or other locations?
See this note from the main page for e-vent.
“Any solution should be utilized only in a healthcare setting with direct monitoring by a clinical professional. While it cannot replace an FDA-approved ICU ventilator, in terms of functionality, flexibility, and clinical efficacy, the MIT E-Vent is anticipated to have utility in helping free up existing supply or in life-or-death situations when there is no other option.”
The e-vent website includes a Testing page. Go through it.
Hi everybody
where can i find the ambubag and its accessories?.
I contacted the company but they answer was “we can not supply it to you”.
Thanks in advance.
my email: waldimar65@hotmail.com
The ET tube is a single tube but is conducting gas flow 2 ways, in and out. The out/exhale has CO2 and should not be mixed with the next breath; that it is called rebreathing and will raise blood CO2. The typical use at the Ambu is direct connection to the ET. Just past this connector elbow is an exhale one way valve allowing the CO2 exhaled breath to escape the breathing system. Most Covid19 systems would put a HEPA filter and PEEP devices next. So if there is a need to place an extension between the Ambu exhalation valve and the ET, this would be dead space (2 way traversing gases with mixing and rebreathing). Yes, spontaneously ventilating patients can be on a high flow ‘T-piece’. Often done in the MRI scanner. But we are not talking about spontaneous ventilation; this is positive pressure (invasive by ET) ventilation. Mapleson described how to deal with dead space, see the intro to this plumbing section. Move the fresh gas or move the exhalation valve are the ways that have proven effective.
What kind of a breathing system have they used? Which Mapleson’s System?
Thanks,
M
Mapleson, see ref at top of page, explains how to get rid of dead space. We have plumbing diagrams above that address this in the context of the E-Vent.
This is the same solution we have come to. One question we have not gotten concrete response from clinicians is whether there is an issue leaving the PEEP valve port on the BVM open as you have. The issue is there is pure oxygen in that line that will escape on every exhale. Also, when drugs are administered to the patient are they injected into the airway such that they would also escape through this opening? From our initial discussions with resp. therapists, it seems they would add the medication as close to the patient as possible (like on the patient side of the HEPA filter). This solves the medication problem. But you still have the issue of oxygen leakage.
Have you received guidance on this issue?
Hi Jacob,
Thanks for your question! Air lost via the exhalation valve at the proximal end of the tubing near the ventilation bag is of equivalent composition to the air input from the bag, so there is not any loss of oxygen from the system. We believe that any loss of air volume from the system is negligible and will be replaced from next cycle tidal volume. A concern could arise from a slight decrease in post-expiratory PIP, but if present it would also be negligible.
How have you solved the leakage problem in the circuit?
So far we have not seen a significant leakage issue; it would show up as an unexplained pressure loss. If components are loose and just won’t tighten up, then some tape to secure them should do the trick. In the end, the patient is still a decent sensor.
Good morning everyone. I am looking after the HEPA Filter specs but I haven´t found any. May you please give me some assistance on that?
My best regards!
Eduardo Viana
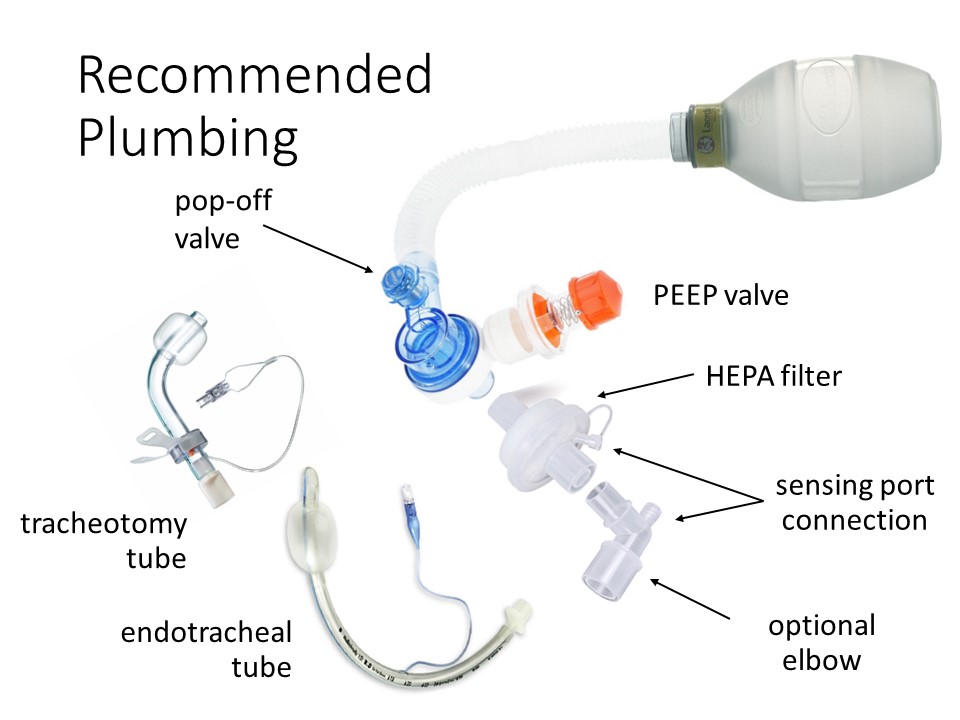
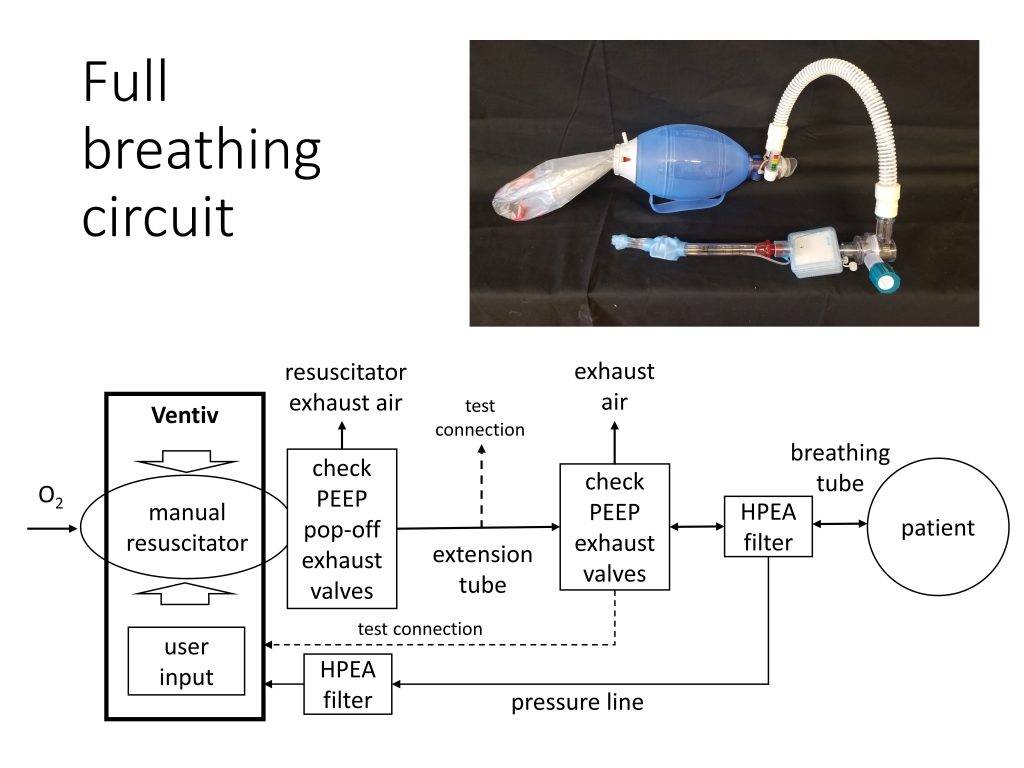
Does a Bill of Materials exist for the Full Circuit Recommended Plumbing shown?
Hello. Can someone assist as I am looking for the recommended component part numbers for the circuit as shown and where these components can be purchased? Thanks in advance!
hello people
i am from brazil and we are working hard for new ventilators for this pandemia.
I wanna know how and where is the exhaust system? because i have some difficulties in understand how is the exausth? another problem for me: you put an HEPA in the in/outlet gas trachea?
sorry by my poor english
Felipe,
Please read the Plumbing portion of the e-vent at https://e-vent.mit.edu/mechanical/plumbing/
Search for “exhal” and also see a picture that includes a HEPA filter before any exhaled air goes out to the environment. Ingress air is also filtered if the HEPA filter is positioned correctly. The URL also points out that adding a HEPA filter may reduce the measured pressure for air flow to the patient.
Hi there!
Something is not quite clear for me, yet. There is a section in this website about non-invasive ventilation, but I see an ET in this section. Is the MIT E-Vent designed for invasive ventilation, non-invasive ventilation or both?
Thank you very much
Hi, MIT E-Vent is strictly designed for invasive ventilation. Non-invasive ventilation is just to give people more clinical context especially to those without medical background.
If an oxygen tank is used with e-vent, the oxygen tank includes a pressure sensor. See
https://www.fierceelectronics.com/components/pressure-and-oxygen-sensors-oxygen-concentrators
The e-vent has its own pressure sensor. Won’t you have to use a T connector to send one pipe to oxygen sensor and another to the e-vent sensor? Does using two sensors instead of one reduce any pressure and report incorrect readings to both sensors? Have we tested the e-vent with an external oxygen tank with merged air flow yet?
Hi, you guys are doing great work here. I was wondering how you sourced all the fittings and connections required for the plumbing. Are they available commercially, or did you have to reach out to specific entities for access to such parts? Thank you.
Please see the “Clinical” page on the E-Vent website for information about the specific components (Link: https://e-vent.mit.edu/clinical/).
Hi, great work guys! This is really impressive.
Do you have a bill of materials available for the plumbing?
Thank you!
Hi,
Pls explain me (in detail) how the differential pressure sensor is connected.
Thanks you!
Hi
I have single breathing circuit. What is the correct way to connect with ambu bag
Thanks
Thanks MIT team for this website! All the information here helped me a lot in building my own ventilator.
This the one I built: http://magicventilator.com
What you think of it?